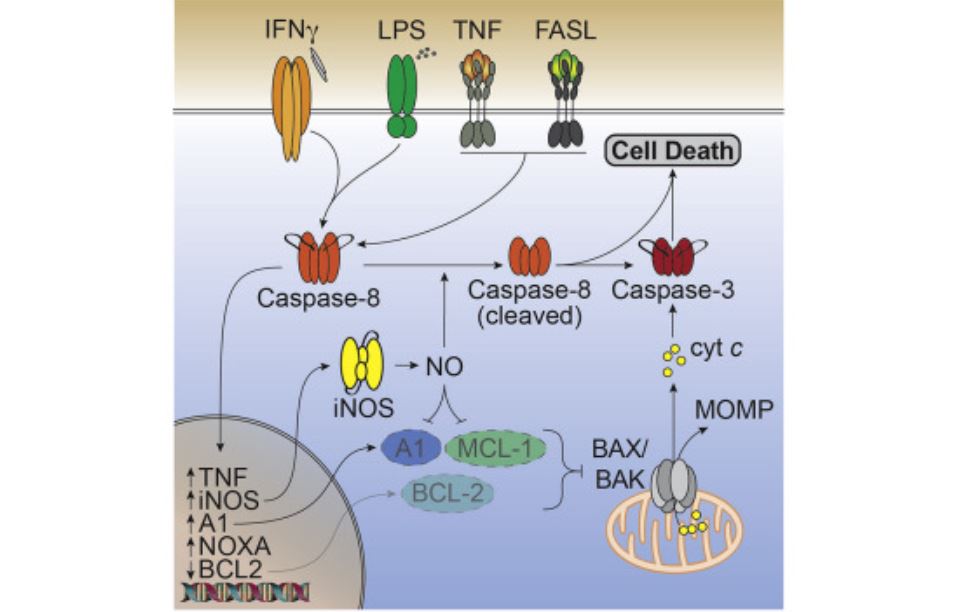
•IFNγ and TLR signaling causes cell death via caspase-8, iNOS, and BAX/BAK
•Caspase-8 regulates BCL2 and iNOS expression to activate BAX/BAK independent of BID
•iNOS causes caspase-8 cleavage and destabilizes the BAX/BAK inhibitors MCL1 and A1
•Caspase-8 and iNOS promote severe disease upon SARS-CoV-2 infection of mice
A WEHI-led study has identified a molecular ‘culprit’ responsible for causing damaging levels of cell death and inflammation in the body. The findings could lead to improved treatment options for a range of conditions driven by inflammatory cell death, including the SARS-CoV-2 virus.
Cell death is an important part of the body’s immune response to infection. When uncontrolled, however, it can cause harmful amounts of inflammation in otherwise healthy organs and tissue. The research team uncovered how an overproduction of the molecule nitric oxide, which the protein caspase-8 helps to produce, caused dangerous levels of cell death. They showed that arresting the function of caspase-8 could prevent unregulated cell death and inflammation.
Published in Immunity, the findings highlight the potential to create drugs that block caspase-8 and nitric oxide to prevent this novel inflammatory cell death process. Manipulating this cell death pathway could lead to new and improved treatments for people living with inflammatory disease.
The study was led by PhD student Daniel Simpson, Associate Professor James Vince and Dr Rebecca Feltham from WEHI, in collaboration with researchers from Monash University, Australian National University, the Hudson Institute of Medical Research and Germany’s Cologne University.
Nitric oxide and the protein that enables its production, caspase-8, have been shown to cause a unique form of cell death that can drive excessive levels of inflammation in the body.
The team showed that blocking the activity of caspase-8 and nitric oxide in a preclinical SARS-CoV-2 model reduced the severity of inflammation and infection.
The findings suggest targeting this novel cell death pathway could create new therapeutics for a range of diseases where damaging levels of nitric oxide, cell death and inflammation occur including asthma, inflammatory bowel disease and COVID-19.
While nitric oxide is critical to the body’s circulatory and nervous systems, the recent findings link an overproduction of the molecule with excessive levels of cell death and inflammation. Cell death is critical for a healthy immune response, however, too much of it can send the immune system into overdrive and trigger inflammatory disease.
Associate Professor James Vince said the team was surprised to uncover that nitric oxide was a ‘killer culprit’ responsible for driving excessive cell death in the newly discovered inflammatory cell death pathway.
“Our research into the combined actions of pathogen and host inflammatory molecules in the cell death process led us directly to nitric oxide,” Associate Professor Vince said.
“This led us to discover how nitric oxide is the major driving force of cell death in this particular pathway. Our study showed levels of this molecule ramped up when immune cells sensed viral and pathogenic threats. The more nitric oxide that was made, the more likely it was that cells would die.”
COVID-19 implications
Collaborating with WEHI infectious disease researchers Professor Marc Pellegrini, PhD student James Cooney, Dr Marcel Doerflinger and Dr Kathryn Davidson, the team found that blocking the production of nitric oxide and the cell death protein caspase-8 reduced the severity of disease in a preclinical SARS-CoV-2 infection model.
Lead researcher and PhD student Daniel Simpson said removing caspase-8 or nitric oxide in these models most likely stops cells from dying and causing tissue damage, highlighting the potential to use caspase-8 as a drug target that could block excessive cell death and the subsequent inflammatory response.
“While this is still preliminary data, we believe blocking the function of caspase-8, or the production of nitric oxide, would prevent damaging levels of inflammation,” he said.
The team leveraged WEHI’s CRISPR technology systems to genetically dissect the new cell death pathway and further understand the role of the key genes involved.
Dr Rebecca Feltham said that DNA editing technology was used to create mutations in genes to determine which genes facilitated nitric oxide production in this cell death pathway.
“Coupled with our COVID-19 models, this technology allowed us to understand the exact role of caspase-8. Being able to understand and manipulate key genes in this pathway could lead to exciting new treatment options for diseases where damaging nitric oxide has been suggested to occur such as asthma, inflammatory bowel disease and the SARS-CoV-2 virus,” she said.
The research was supported by NHMRC, the German Research Foundation, the Leukemia and Lymphoma Society and the Australian Research Council. Wehi authors: Daniel Simpson, Jiyi Pang, Ashley Weir, Isabella Kong, Maryam Rashidi, James Cooney, Kathryn Davidson, Sebastian Hughes, Liana Mackiewicz, Merle Dayton,Holly Anderton, Marcel Doerflinger,Yexuan Deng, Allan Shuai Huang, Sandra Nicholson, Joanna Groom, Marco Herold, Edwin Hawkins, Andreas Strasser, John Silke, Marc Pellegrini and Rebecca Feltham.
https://www.wehi.edu.au/news/molecular-culprit-caught-driving-unwanted-cell-death






Recent Comments