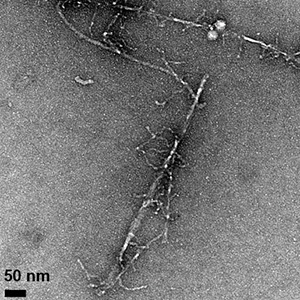
Fibrils formed by the aggregation of the amyloid beta protein can be seen in these transmission electron microscope images, which show differences in fibril morphology between the normal protein (above) and an altered protein with one amino acid replaced by its mirror image. The altered protein also forms fibrils more slowly and is more toxic to cells. (Image credit: Warner et al., CEJ 2016)
Subtle change to amyloid beta protein affects its aggregation behavior, stabilizes an intermediate form with enhanced toxicity. Much of the research on Alzheimer’s disease has focused on the amyloid beta protein, which clumps together into sticky fibrils that form deposits in the brains of people with the disease. In recent years, attention has turned away from the fibrils themselves to an intermediate stage in the aggregation of amyloid beta. “Oligomers” consisting of a few molecules of the protein stuck together are more mobile than the large, insoluble fibrils and seem to be much more toxic. But the structure of these soluble oligomers remains unknown, and it’s unclear how they trigger the neurotoxicity.
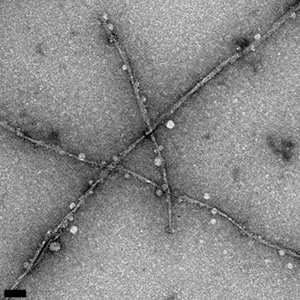
UC Santa Cruz researchers made a subtle alteration to the amyloid beta protein that had striking effects on its properties. By replacing one amino acid with its mirror image, they created a version of amyloid beta with a reduced rate of fibril formation, different fibril structure, and increased toxicity in cell culture compared to the normal or “wild type” protein.
Most amino acids can occur in 2 mirror-image forms, a “left-handed” or L-form and a “right-handed” or D-form, but living cells only make proteins out of L-amino acids. Raskatov’s team changed one amino acid in the amyloid beta protein to its D-form: the glutamate at position 22. “This subtle change stabilizes a prefibrillary intermediate that has higher toxicity,” he said, noting that the stable intermediate could be a very useful tool for investigating the neurotoxic effects of amyloid beta oligomers. With a better understanding of the molecular mechanisms researchers hope to identify new targets for drug development.
Several mutations associated with inherited early-onset Alzheimer’s disease affect position 22 in the amyloid beta protein, either changing it from glutamate to another amino acid or deleting it. Using small-angle xray scattering analysis, they found evidence that the protein forms a soluble oligomer with an ordered structure. http://news.ucsc.edu/2016/06/alzheimers-disease.html






Recent Comments