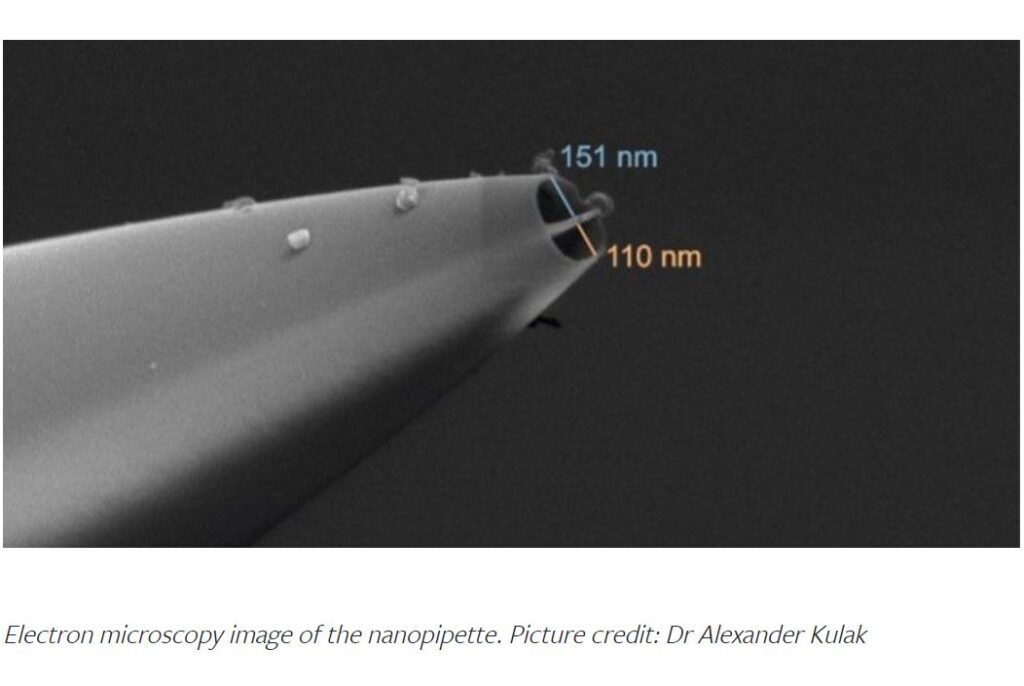
A groundbreaking nanosurgical tool — about 500 times thinner than a human hair — could be transformative for cancer research and give insights into treatment resistance that no other technology has been able to do, according to a new study.
The high-tech double-barrel nanopipette, developed by University of Leeds scientists, and applied to the global medical challenge of cancer, has — for the first time — enabled researchers to see how individual living cancer cells react to treatment and change over time — providing vital understanding that could help doctors develop more effective cancer medication.
The tool has two nanoscopic needles, meaning it can simultaneously inject and extract a sample from the same cell, expanding its potential uses. And the platform’s high level of semi-automation has sped up the process dramatically, enabling scientists to extract data from many more individual cells, with far greater accuracy and efficiency than previously possible, the study shows.
Currently, techniques for studying single cells usually destroy them, meaning a cell can be studied either before treatment, or after.
This device can take a “biopsy” of a living cell repeatedly during exposure to cancer treatment, sampling tiny extracts of its contents without killing it, enabling scientists to observe its reaction over time.
During the study, the multi-disciplinary team, featuring biologists and engineers, tested cancer cells’ resistance to chemotherapy and radiotherapy using glioblastoma (GBM) — the deadliest form of brain tumour — as a test case, because of its ability to adapt to treatment and survive.
Their findings are published March 6 in the journal Science Advances.
Significant breakthrough
One of the paper’s corresponding authors, Dr Lucy Stead, Associate Professor of Brain Cancer Biology in the University of Leeds’ School of Medicine, said: “This is a significant breakthrough. It is the first time that we have a technology where we can actually monitor the changes taking place after treatment, rather than just assume them.
“This type of technology is going to provide a layer of understanding that we have simply never had before. And that new understanding and insight will lead to new weapons in our armoury against all types of cancer.”
She added: “GBM is the cancer in most need of those new weapons because in 20 years there has been no improvement in survival in this disease.
“It is lagging behind so much and we think that is because of the highly ‘plastic’ nature of these tumours — their ability to adapt to treatment and survive it.
“That is why it is so important that we can dynamically observe and characterise these cells as they change, so we can map out the journey these cells can take, and subsequently find ways to stop them at every turn. We simply couldn’t do that with the technologies that we had.”
Transformative
Dr Stead leads the Glioma Genomics research group at the Leeds Institute of Medical Research at St James’s Hospital, which is focused on trying to cure GBM brain tumours. She added: “This technology could be transformative for this particular cancer, helping us finally identify effective treatments for this awful, incurable disease.”
The research was primarily funded by The Brain Tumour Charity, which counts former Leeds footballer Dominic Matteo as one of its high-profile supporters. Matteo did not have GBM but underwent surgery to remove a brain tumour in 2019.
Dr Simon Newman, Chief Scientific Officer at The Brain Tumour Charity, said: “We know glioblastoma cells respond differently to treatment, often developing treatment resistance which leads to recurrence. The development of this novel technology, which can extract samples from tumour cells grown in the lab before and after treatment, will give a unique insight into how drug resistance may develop and lead to tumours growing back.
“We hope that this important work, funded by The Brain Tumour Charity, will improve our knowledge of these complex brain tumours and allow us to find new, more effective treatments — something so urgently needed for those faced with this devastating disease.”
Collaborative
The study was a collaboration between researchers from Leeds’ Bragg Centre for Materials Research; Leeds’ School of Electronic and Electrical Engineering; Leeds Institute of Medical Research, and the Earlham Institute, Norwich, who studied single GBM cells over a period of 72hrs.
They used the nanosurgical platform, which is far too small to be manipulated by hand. The miniscule needles are precisely controlled by robotic software to manoeuvre them into position, into the cells in the petri dish. The nanopipette’s second needle plays a fundamental role in controlling the equipment.
The device allows scientists to take samples repeatedly, to study the progression of disease in an individual cell. Much research on molecular biology is carried out on populations of cells, giving an average result that ignores the fact that every cell is different.
Some cells die during treatment, but others survive. The key to finding a cure is understanding what allows one cell to survive and what is happening to the ones that die.
Unprecedented precision
Lead author Dr Fabio Marcuccio, Research Associate in the Faculty of Medicine at Imperial College London, who carried out the research while at Leeds, said: “Our device allows the study of the way brain cancer cells adapt to treatment over time, with unprecedented precision. This tool will provide data that could lead to significant improvements in cancer treatment and prognoses.”
He added: “This work is the result of a collaborative effort with my colleagues and co-leads Dr Chalmers Chau, Research Fellow in Bionanotechnology in Leeds’ School of Electronic and Electrical Engineering, and Dr Georgette Tanner, formerly of Leeds, now Bioinformatician at Oxford Nanopore Technologies, whose contributions were fundamental to the experimental design and data analysis. This demonstrates the importance of creating an interdisciplinary team to tackle the biggest challenges of our time.”
Cancer cell plasticity — the ability of cells to change their behaviours — is one of the biggest challenges in cancer treatment as it remains poorly understood. GBM cancer cells are particularly “plastic”: they can adapt very quickly, and this is thought to help them develop resistance to radiotherapy and chemotherapy. Learning how these cells adapt, and subsequently how we can block them, could prevent cancer from recurring, something which almost always happens with GBM.
Camilla Hawkins, an occupational therapist from London, was diagnosed with GBM in August 2022. The 55-year-old said: “Any findings, such as these, that could help inform new treatments, has got to be welcomed. Extended good quality of life is worth living, even where the prognosis is terminal.”
Crucially important
The other corresponding author and co-lead Dr Paolo Actis, Associate Professor of Bio-Nanotechnology in Leeds’ School of Electronic and Electrical Engineering, has been working on the nanobiopsy tool for around 15 years and said its new capabilities, compared to its original scope, provided “remarkable advantages.”
He added: “Cancer cells that are not killed by chemotherapy are the ones that make the cancer grow back and lead to death.
“Our tool can pinpoint these cells and we can now perform biopsies on them so we can specifically study how the ones that survive treatment have changed.
“This is crucially important as the more we can understand how the cells change, the more drugs we can develop to stop them from adapting.”
Dr Stead said further research needed to be carried out, using this technology on many more samples in the lab and in humans, but that it had already yielded hugely valuable information.
Additional funding was provided by UK Research and Innovation and the European Commission. https://www.leeds.ac.uk/main-index/news/article/5525/nanosurgical-tool-could-be-key-to-cancer-breakthrough









Recent Comments