James Bibb, Ph.D., and colleagues have described a novel preclinical drug that could have the potential to combat depression, brain injury and diseases that impair cognition. The drug, which notably is brain-permeable, acts to inhibit the kinase enzyme Cdk5.
Cdk5 is a crucial regulator of signaling in brain neurons. Over three decades of study, it has been implicated in neuropsychiatric and neurodegenerative conditions, including Alzheimer’s disease and Parkinson’s disease. Knocking out the enzyme in mice makes them resilient to stress, enhances their cognition, protects neurons from stroke and head trauma, and lessens neurodegeneration.
While inhibitors of Cdk5 could offer potential therapeutic benefits and new ways to study basic brain function, previous first- and second-generation anti-Cdk5 compounds largely get blocked at the blood-brain barrier that restricts movement of solutes from the blood to the central nervous system’s extracellular fluid. To date, no Cdk5 inhibitor has been approved to treat any neuropsychiatric or degenerative diseases.
Bibb and colleagues now report details of their anti-Cdk5, brain-permeable compound, 25-106. They also show that systemic administration of 25-106 alters neurobehavior in mice, reducing anxiety-like behavior.
“As perhaps the first robust systemic inhibitor, 25-106 represents an exciting and expandable and translatable pharmacological tool to study the function of Cdk5 activity in wild-type animals,” said Bibb, a professor in the University of Alabama at Birmingham Department of Surgery. “Achieving systemic applicability may be considered a step forward toward the testing of Cdk5 inhibitors to treat neuropsychiatric and neurodegenerative diseases. This provides a promising landscape for future studies to assess the effects of brain-permeable Cdk5 inhibitors to combat stress, anxiety, depression, addiction, cancer and neurodegeneration.”
The study, “Systemic administration of a brain permeable Cdk5 inhibitor alters neurobehavior,” is published in the journal Frontiers in Pharmacology.
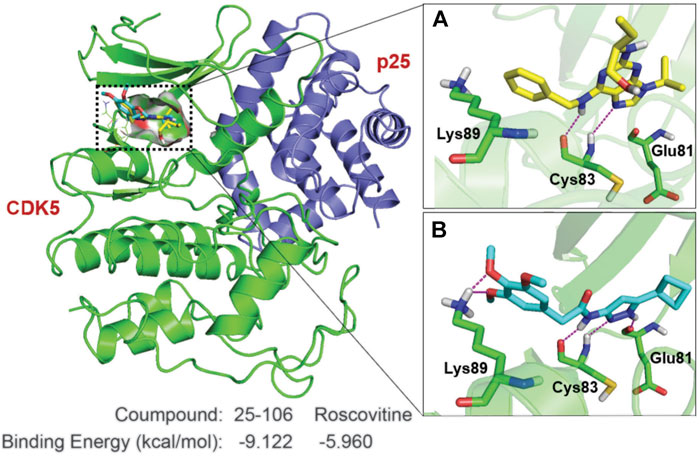
In the paper, researchers describe synthesis of the aminopyrazole-based inhibitor, and they used molecular modeling to show that 25-106 appears to occupy the same hydrophobic binding pocket as the well-established Cdk5 inhibitor roscovitine.
They showed that 25-106 inhibited Cdk5 activity in a dose-dependent manner in brain striatal slices ex vivo, and that it also penetrated the brain after systemic administration in mice to inhibit Cdk5 in vivo. They measured the pharmacokinetic and pharmacodynamic parameters of 25-106 in blood plasma and the brains of mice, and the off-target distribution of 25-106 in the liver and kidneys.
Mice given systemic 25-106 showed modulated neurobehavior in the open field maze test and the tail suspension test, anxiolytic changes that have previously been linked to Cdk5 knockout mice.
They found that 25-106 is a non-selective inhibitor of both Cdk5 and another cyclin-dependent kinase, Cdk2, but note that very low levels of Cdk2 are found in the brain. However, any off-target or toxic effects of systemic inhibition of Cdk2 by 25-106 remain unknown.
According to Bibb, three teams of researchers were involved in this study, including UAB Marnix E. Heersink School of Medicine Department of Surgery neurobiologists Bibb, Alan Umfress and Ayanabha Chakraborti, as well as Florian Plattner of Neuro-Research Strategies, Houston, Texas. Also involved were UAB Department of Pharmacology and Toxicology researchers Kevin J. Ryan and Edward P. Acosta. The third group was composed of University of Nebraska Medical Center medicinal chemists Sarbjit Singh, Yogesh Sonawane, Jayapal Reddy Mallareddy and Amarnath Natarajan.
Support came from National Institutes of Health grants MH116896, MH126948, CA127297 and NS061788.
At UAB, Bibb holds the Dr. Champ Lyons Endowed Chair in General Surgery and is a senior scientist in the O’Neal Comprehensive Cancer Center. https://www.uab.edu/news/research/item/12861-novel-preclinical-drug-could-have-potential-to-combat-depression-brain-injury-and-cognitive-disorders
https://www.frontiersin.org/articles/10.3389/fphar.2022.863762/full






Recent Comments