Long-term studies of ozone and water vapor in the atmosphere of Mars could lead to better understanding of atmospheric chemistry for the Earth. A new analysis of data from ESA’s Mars Express mission has revealed that our knowledge of the way these atmospheric gases interact with each other is incomplete.
Using four martian years of observations from the SPICAM (Spectroscopy for the Investigation of the Characteristics of the Atmosphere of Mars) instrument, which corresponds to seven and a half Earth years, a team of researchers from Europe and Russia uncovered the gap in our knowledge when trying to reproduce their data with a global climate model of Mars.
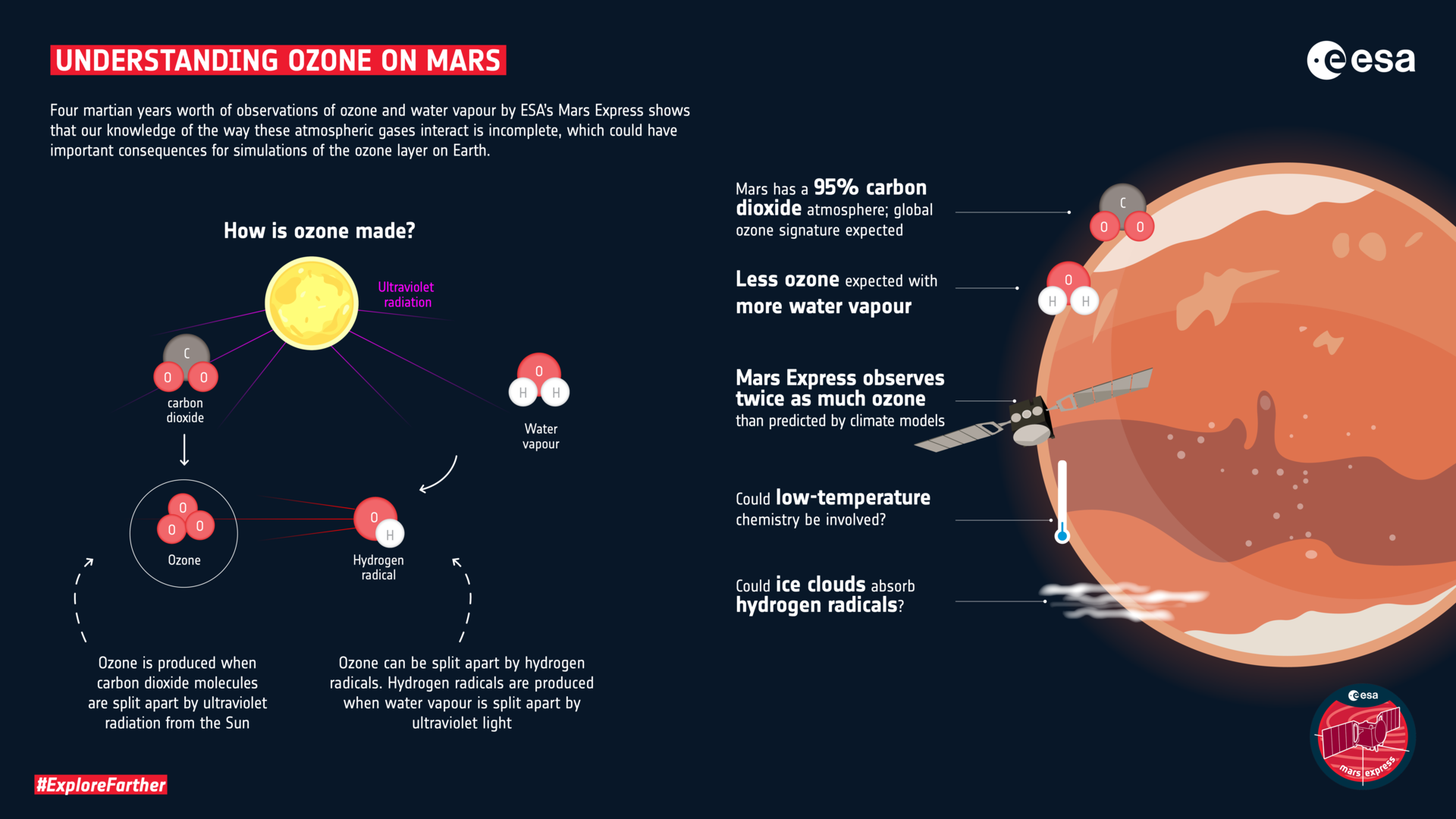
Ozone and water vapor do not make good atmospheric companions. The ozone (O3) is produced when molecules of carbon dioxide (CO2), which comprises 95% of the martian atmosphere, are split apart by ultraviolet radiation from the Sun. In turn, the ozone can be split apart by molecules called hydrogen radicals (HOX), which contain an atom of hydrogen and one or more atoms of oxygen. The hydrogen radicals themselves are produced when water vapor is split apart by ultraviolet light.
On Mars, since the carbon dioxide is ubiquitous, there should be a global signature of ozone—unless a particular region contains water vapor. In that circumstance, the water will be split into hydrogen radicals, which will react with the ozone molecule and pull it apart.
Thus, wherever SPICAM detected water vapor, it should have seen a decrease in ozone. The more water vapor, the less ozone. The team investigated this inverse relationship, also known as an anticorrelation. They found that they could reproduce the general inverse nature of it with a climate model but not achieve the precise relationship. Instead, for a given amount of water vapor, the model produced only 50% of the ozone seen in the SPICAM data.
“It suggests that the efficiency of ozone destruction is overstated in the computer simulations,” says Franck Lefèvre, of the Laboratoire atmosphères, milieux, observations spatiales (LATMOS), CNRS/Sorbonne Université, France, who led the study.
At present, however, the reason for this over-estimation is not clear. Understanding the behavior of hydrogen radicals on Mars is essential. “It plays a key role in the atmospheric chemistry of Mars but also in the global composition of the planet,” says Franck.
The chemical model used in this work was built specifically by Franck and colleagues to analyze Mars. It was based on a model of part of the Earth’s upper atmosphere; the mesosphere. Here, between roughly 40-80 kilometers in altitude, the chemistry and conditions are broadly similar to those found in Mars’s atmosphere.
Indeed, the discrepancy found in the models could have important repercussions for the way we simulate the Earth’s climate using atmospheric models. This is because the mesosphere on Earth contains part of the ozone layer, which will experience the same interactions with HOX as take place on Mars.
“HOX chemistry is important for the global equilibrium of the Earth’s ozone layer,” says Franck.
So, understanding what is happening in the atmosphere of Mars could benefit the precision with which we can perform climate simulations on Earth. And with so much data now available from SPICAM, the modeling has clearly shown that there is something we don’t understand.
Could that something be the action of clouds?
When Frank’s colleagues introduced calculations for the way HOX is absorbed by the icy particles that make up clouds on Mars, they found that more ozone survived in their models. This is because HOX molecules were absorbed before they could pull apart the ozone. But this only partially explained their results.
“It doesn’t work in all the cases,” says Franck. And so the team are looking elsewhere too.
One particular area for further study is measuring reaction rates at the low temperatures found in the martian atmosphere and Earth’s mesosphere. At present, these are not well known, and so could also be skewing the models.
Now that the current work has highlighted in a quantitative way where the gaps lie in our knowledge, the team will collect more data using other UV instruments operating at Mars and continue their investigations and update the model.
“With Mars Express, we have a completed the longest survey of the martian atmosphere to date, regardless of the mission. We started in 2004, and now have 17 years of data, which has led us to look at almost seven martian years in a row, including four martian years of combined ozone and water vapor measurements before the UV channel of SPICAM, which measured ozone, ceased operating near the end of 2014. This is unique in the story of planetary exploration,” adds Franck Montmessin, also from LATMOS, and the principal investigator of the SPICAM instrument.
Building on the extraordinary dataset from Mars Express, new results are now coming in from ESA’s Trace Gas Orbiter, which has been circling Mars since October 2016. It carries two instruments, ACS (Atmospheric Chemistry Suite) and NOMAD (Nadir and Occultation for MArs Discovery) that are analyzing the martian atmosphere. NASA’s Maven mission also carries ultraviolet equipment that monitors ozone abundance. So, the vital piece of information that finally unlocks this mystery could come at any time.
The long-term monitoring of atmospheric parameters and their variations by Mars Express provides a unique data set with which to study the martian atmosphere as a complex dynamic system.
“Maybe adding up all these years together will eventually hold the key to how the HOX really controls the martian atmosphere, benefiting our understanding of planetary atmospheres in general,” says Franck Montmessin.






Recent Comments