SEBACIC ACID
~ also known as decanedioic acid; 111-20-6; 1,8-Octanedicarboxylic acid; 1,10-Decanedioic acid. Sebacic acid is a saturated, straight-chain naturally occurring dicarboxylic acid with 10 carbon atoms and is a normal urinary acid. Sebacic Acid Market size was above USD 300 million in 2016 and consumption might surpass 100 kilo tons by 2024. 90% of world production occurs in China.
Chemical structure: HOOC(CH2)8COOH or C10H18O4
Properties: White flake or granular powder. Melting point 153°F. Slightly soluble in water and sublimes slowly at 750 mm Hg at melting point.
Production: Sebacic acid can be made from phenols and cresols, but castor oil oxidation is a greener process. Byproducts include glycerol, octanol and polymer residue.
a) Castor oil is heated to about 250oC with alkali => results in saponification to ricinoleic acid => cleaved to 2-octanol and sebacic acid
b) Adipic acid electro oxidation involves partial esterification to monomethyl adipate. Electrolysis of the potassium salt of monomethyl adipate in a methanol/ water mixture produces dimethyl sebacate => undergoes hydrolysis to sebacic acid.
Chemical Reactions and Uses: Sebacic Acid and its derivatives e.g. azaleic acid are primarily used in production of plasticizers, lubricants, hydraulic fluids, anti-freeze, de-icing products, cosmetics, candles, etc. It is used in the synthesis of polyamide and alkyd resins. It is also an intermediate for aromatics, antiseptics and painting materials.
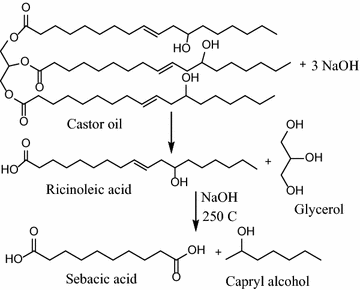
Image from sustainablechemicalprocesses.springeropen.com
>> see the worldwide chemical supplier marketplace of sebacic acid and much more







Recent Comments