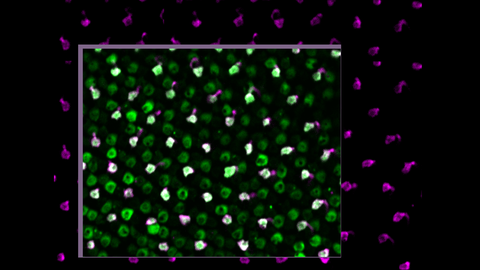
Blinding diseases lead to permanent vision loss by damaging photoreceptor cells, which humans cannot naturally regenerate. While researchers are working on new methods to replace or regenerate these cells, the crucial question is whether these regenerated photoreceptors can fully restore vision. Now, a team of researchers led by Prof. Michael Brand at the Center for Regenerative Therapies Dresden (CRTD) of Dresden University of Technology has made an important step forward. By studying zebrafish, an animal naturally capable of photoreceptor regeneration, the team showed that regenerated photoreceptors are as good as original ones and regain their normal function, allowing the fish to recover complete vision. Their results, published in the journal “Developmental Cell,” offer promising insights for the future of photoreceptor replacement therapies.
Vision is a complex sense that depends on the retina. This complex neural tissue in the back of our eyes is actually an external piece of the brain. It is where photoreceptor cells capture light and convert it into electrical signals. For humans, these photoreceptors are not replaced after damage. Once lost, they do not regenerate, leading to irreversible vision loss.
Therapies that are currently under development, including at the CRTD in Dresden, aim to replace damaged human photoreceptors and restore vision, either by stimulating stem cells within the retina to develop into new photoreceptors or by transplanting photoreceptors grown outside of the body.
Unlike humans, zebrafish have a remarkable ability to regenerate parts of their nervous system even after severe damage. Zebrafish can regrow photoreceptors from special stem cells located in the retina, known as Müller glia. This unique ability makes zebrafish an ideal model for studying the potential to restore vision through photoreceptor regeneration.
“Mammalian retina, including human retina, has very similar Müller glia cells. However, our cells have lost the ability to regenerate during evolution. Since these cells are so very similar, however, it may be possible to rekindle this regeneration potential for therapeutic applications in the future,” says Prof. Michael Brand, research group leader at the CRTD who led the study. “However, it is crucial to determine if such new photoreceptor cells can function as effectively as the originals.”
Making the Impossible Measurements
Researchers have long known that zebrafish can regenerate damaged retinas, with new photoreceptors appearing identical to the originals. Various groups, including the group of Prof. Brand, developed behavioral tests that confirmed that fish regained vision after regeneration. But these tests could not directly assess the extent to which the photoreceptor function was restored.
“The only comprehensive test to see if the vision is fully restored is to directly measure the electrophysiological activity of the retinal cells. Are photoreceptors correctly stimulated by the various colors of light? Are they electrically active to the same extent? Are they connected to the neighboring cells? Are they passing the signal to them? Are all the typical circuits engaged?” says Prof. Brand.
To answer these questions, the Brand team used a genetically modified zebrafish that let them use high-end microscopy to track the activity of photoreceptors at the photoreceptor synapse, i.e., directly where the photoreceptors connect to other nerve cells and pass the electric signal forward.
However, testing the function of regenerated photoreceptors proved to be a significant technical challenge. Photoreceptors convert light into electrical signals. But using light to observe cells under the microscope simultaneously stimulates them. This technical difficulty seemed almost impossible to overcome. However, with input from Prof. Tom Baden from the University of Sussex in Brighton, U.K., and Dr. Hella Hartmann, leader of the Light Microscopy Facility at the Center for Molecular and Cellular Bioengineering at TUD, it was possible to build a custom microscope that allowed the team to uncouple the stimulation from observation and measurement for different light colors, and to overcome this technical hurdle.
Using this advanced custom setup, the Brand team could show that the regenerated photoreceptors indeed regain their normal physiological function. They respond to light at different wavelengths, transmit the electric signal to neighboring cells, and do so with the same sensitivity, quality, and speed as original photoreceptors in an intact retina.
Hope for the Future
“Restoring all of these aspects of photoreceptor function, together with our previous work on restoring vision-controlled behavior, confirmed on a molecular level that the fish can fully ‘see’ again,” says Prof. Brand.
“Humans and fish have a common evolutionary ancestry and share most of the genes and types of cells. Therefore, we hope that humans can learn this ‘regeneration trick’ from the zebrafish. It is important to note that, at this stage, our work is classical basic research. It is still a long way until it can be applied in the clinic. However, being able to eventually achieve such functional regeneration from stem cells already located in the human retina could potentially revolutionize the treatment of currently untreatable diseases like retinitis pigmentosa or macular degeneration. This study brings us one step closer to that dream,” concludes Prof. Brand. https://tu-dresden.de/cmcb/crtd/news-termine/news/seeing-the-future-zebrafish-regenerates-fully-functional-photoreceptor-cells-and-restores-its-vision







Recent Comments