A groundbreaking study published today and led by the Translational Genomics Research Institute (TGen), an affiliate of City of Hope, identifies unique lung cells that may drive Idiopathic Pulmonary Fibrosis (IPF), a deadly lung disease that affects hundreds of thousands of Americans, and for which there is no cure.
This is one of the first comprehensive looks at lung cells using a technology called single-cell RNA sequencing. Instead of examining a mash-up of many cells from a tissue sample, single-cell sequencing allowed researchers in this study to closely examine the individual cells that make up the lungs; to identify their function, and ultimately understand the molecular changes that may be driving the disease.
Using this method, researchers identified five unique cells types associated with lung fibrosis, which could potentially lead to earlier diagnosis and therapeutic drug targets.
The study’s initial findings, published as the cover story for the journal Science Advances, are the first under a combined $6.1 million in federal grants aimed at uncovering the origins of lung disease, including IPF, the nation’s most common and severe form of fibrotic lung disease. An estimated 50,000 Americans, mostly middle-aged and older adults, are diagnosed each year with IPF. Most die from respiratory failure within five years.
In collaboration with Vanderbilt University Medical Center (VUMC), researchers analyzed tissue samples from 20 lungs with pulmonary fibrosis provided by the Norton Thoracic Institute and VUMC, and tissue samples from 10 healthy lungs provided by the Donor Network of Arizona and the Tennessee Donor Services.
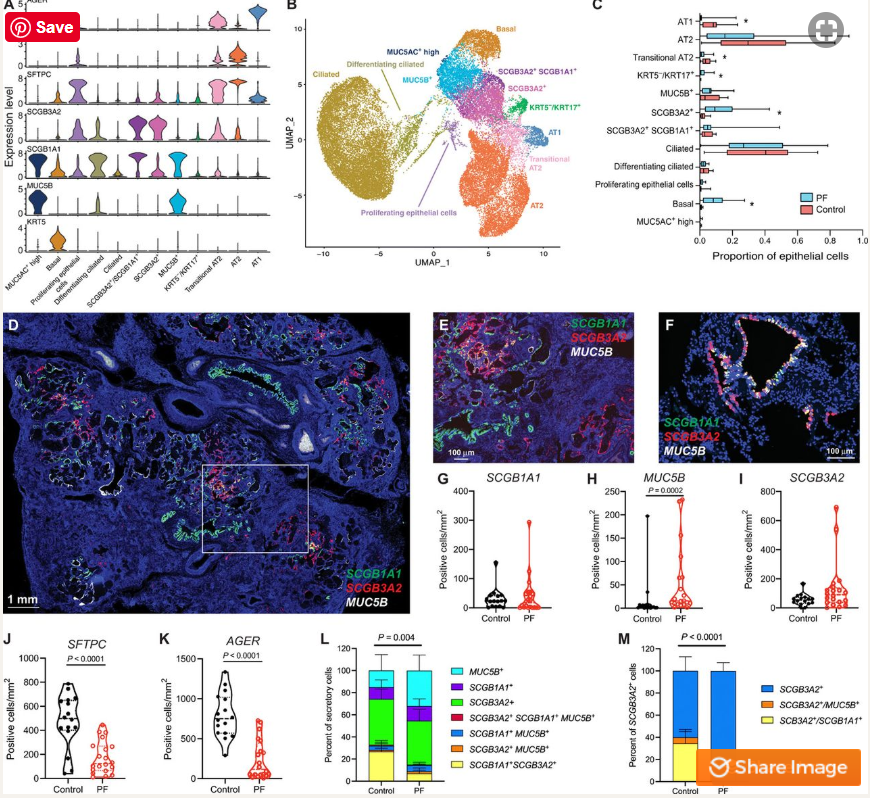
Dr. Nicholas Banovich, an Assistant Professor in TGen’s Integrated Cancer Genomics Division and co-senior author of the study, said the most interesting finding is the characterization of cells called KRT5-/KRT17+, which appeared in the epithelium, or protective lining of the lungs, but only in individuals with pulmonary fibrosis.
“These cells are incredibly unique as they are clearly epithelial, but are also producing collagen and components of extra-cellular matrix (ECM), which make scar tissue,” Dr. Banovich said, “They are directly contributing to fibrosis.” These cells also share characteristics of both the airway and alveolar epithelium, the respiratory membrane that allows the exchange of gases.
Another of the study’s most interesting findings is the high degree of plasticity, the ability of a cell to share characteristics with multiple classically defined cell types, in the lung epithelium.
“Classically, the field used a small number of genes to determine cell types. With the single cell RNA sequencing approach, we find that it is often hard to draw a firm line between different types of cells,” Dr. Banovich said. “Instead of thinking of them as discrete cell types, we should think of them more along a continuum, and given the right stimulus, these cells can change their state.”
In addition to the KRT5-/KRT17+ cells, the study identified a cell type marked by the gene SCGB3A2. These cells are similar to club cells — an epithelial cell that lines the airways — yet are found almost exclusively in in pulmonary fibrosis. Unlike other airway epithelial cells, it appears these cells are able to transform into type 1 alveolar cells (AT1) — the cells where oxygen is taken into the body, and through which carbon dioxide is expelled — in an effort to repair damage to the lung.
“In addition to becoming AT1 cells, our results suggest the SCGB3A2+ cells can also become the KRT5-/KRT17+ cells. It actually appears that, during the transformation into AT1 cells, the process is being hijacked and instead of helping repair the lungs these cells are pushed toward this weird pro-fibrotic epithelial cell that continues to drive fibrosis,” Dr. Banovich said.
Two other cells unveiled by the study are distinct subsets of fibroblasts, the cells that form normal connective tissue and scar tissue in the lungs, marked by high levels of the genes PLIN2 or HAS1. These cells are also limited to pulmonary fibrosis.
IPF is a progressive and irreversible disease characterized by a dry cough, fatigue, aching muscles and joints, and ever worsening shortness of breath. IPF and PF both scar and stiffen the interstitium — the delicate lace-like network that supports the lungs’ tiny air sacs. IPF has both genetic and environmental risk, but the exact cause is unknown and current treatments short of a lung transplant only slow disease progression. Lung transplants are radical surgeries that usually require months of waiting for available organs, and often require a long and sometimes agonizing recovery.
The study acknowledges the help of: 10x Genomics and the Chan Zuckerberg Initiative in developing technology optimizations of the single-cell RNA sequencing; and the patients and organ donors who made this work possible. https://www.tgen.org/news/2020/july/08/tgen-study-identifies-cells-that-may-drive-lung-fibrosis/ https://advances.sciencemag.org/content/6/28/eaba1972







Recent Comments