
Marc Aurel Busche at the two-photon microscope, which allows to visualize nerve cells with high temporal and spatial resolution in the intact brain. Credit: Copyrighted image: Kurt Bauer / Technical University of Munich
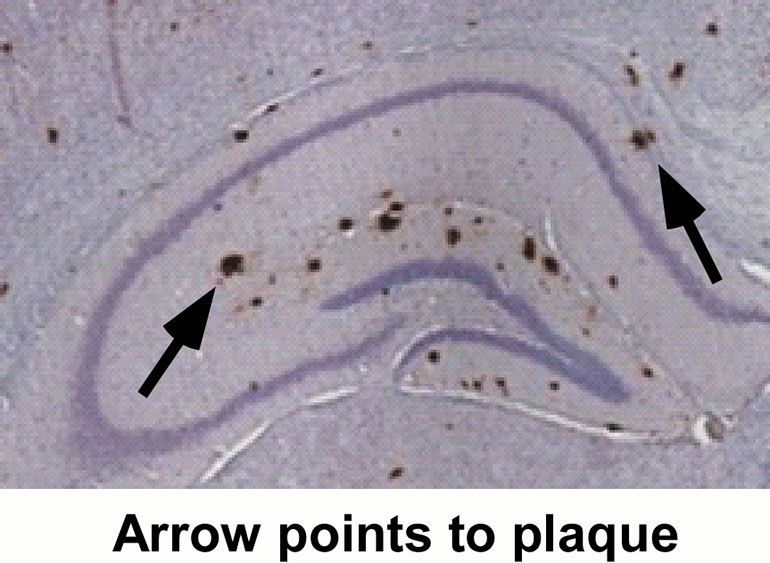
BACE inhibitor successfully tested in Alzheimer’s animal model. The protein amyloid beta is believed to be the major cause of Alzheimer’s disease. Substances that reduce the production of amyloid beta, such as BACE inhibitors, are therefore promising candidates for new drug treatments. A team at the Technical University of Munich (TUM) has recently demonstrated that one such BACE inhibitor reduces the amount of amyloid beta in the brain. By doing so, it can restore the normal function of nerve cells and significantly improve memory performance.
Around 50 million people worl...
Read More






Recent Comments