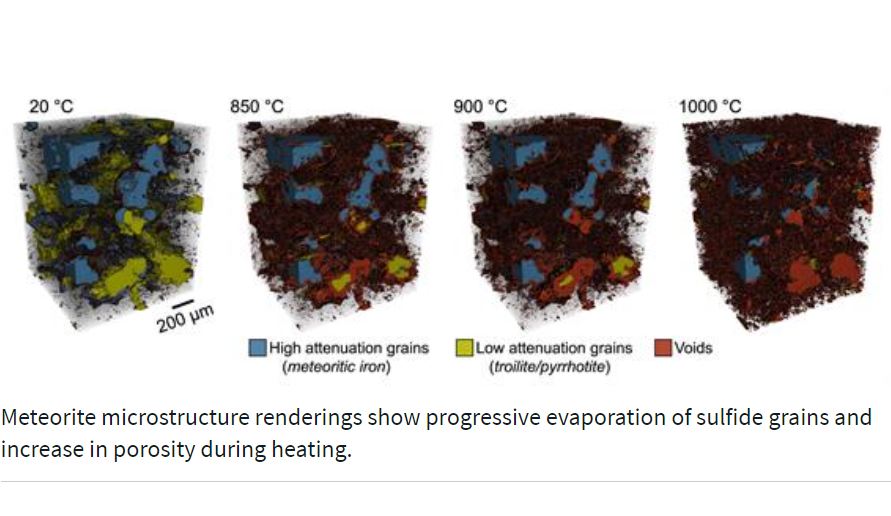
Researchers at the University of Illinois Urbana-Champaign watched fragments of two meteors as they ramped up the heat from room temperature to the temperature it reaches as it enters Earth’s atmosphere and made a significant discovery. The vaporized iron sulfide leaves behind voids, making the material more porous. This information will help when predicting the weight of a meteor, its likelihood to break apart, and the subsequent damage assessment if it should land.
“We extracted samples from the interiors that had not already been exposed to the high heat of the entry environment,” said Francesco Panerai, professor in the Department of Aerospace Engineering at UIUC. “We wanted to understand how the microstructure of a meteorite changes as it travels through the atmosphere.”
Pa...
Read More





Recent Comments