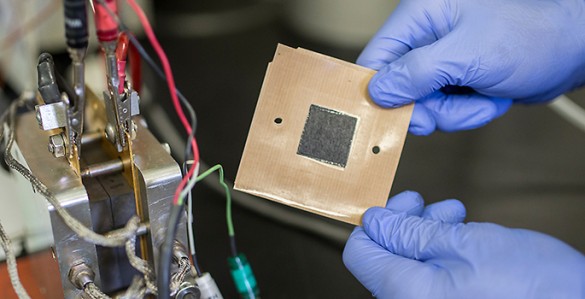
Nanofiber mat electrode (John Russell / Vanderbilt)
The project is part of a $13 million Department of Energy program to advance fuel cell performance and durability and hydrogen storage technologies announced last month. The $4.5 million collaboration is based on a new nanofiber mat technology developed by Peter Pintauro, the H. Eugene McBrayer Professor of Chemical Engineering at Vanderbilt, that replaces the conventional electrodes used in fuel cells. The nanofiber electrodes boost the power output of fuel cells by 30% while being less expensive and more durable than conventional catalyst layers...
Read More







Recent Comments