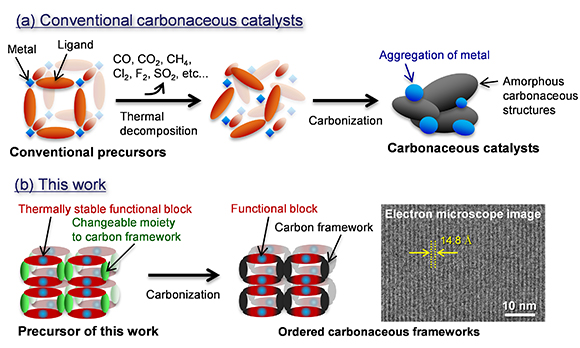
Synthesis schemes of (a) conventional carbonaceous catalysts and (b) this work for ordered carbonaceous frameworks. Credit: Copyright: Hirotomo Nishihara
Researchers have developed a new synthesis route for alternative catalysts of noble metals for versatile chemical reactions that could help address environmental concerns. Noble metals such as platinum are useful as catalysts for versatile chemical reactions including fuel cell vehicles and reduction of CO2 emission. However, they are too costly to be used for these purposes.
As inexpensive alternatives, organic-based catalysts and carbonaceous catalysts were explored, but were ultimately found to be impractical...
Read More






Recent Comments