A genome-editing strategy developed at Rice University can correct dozens of errors at the same time with high precision and efficiency, a possible breakthrough for those who suffer from diseases caused by a combination of mutations.
The “drive-and-process” array, DAP for short, also appears to be highly adept at avoiding off-target edits, errors that have plagued earlier gene-editing strategies.
Engineer Xue Sherry Gao and graduate student and lead author Qichen Yuan of Rice’s George R. Brown School of Engineering introduced their unique CRISPR array architecture in Nature Communications.
Their technique could both simplify and advance the art of gene editing, not only for human diseases but also for basic biology research and crop engineering.
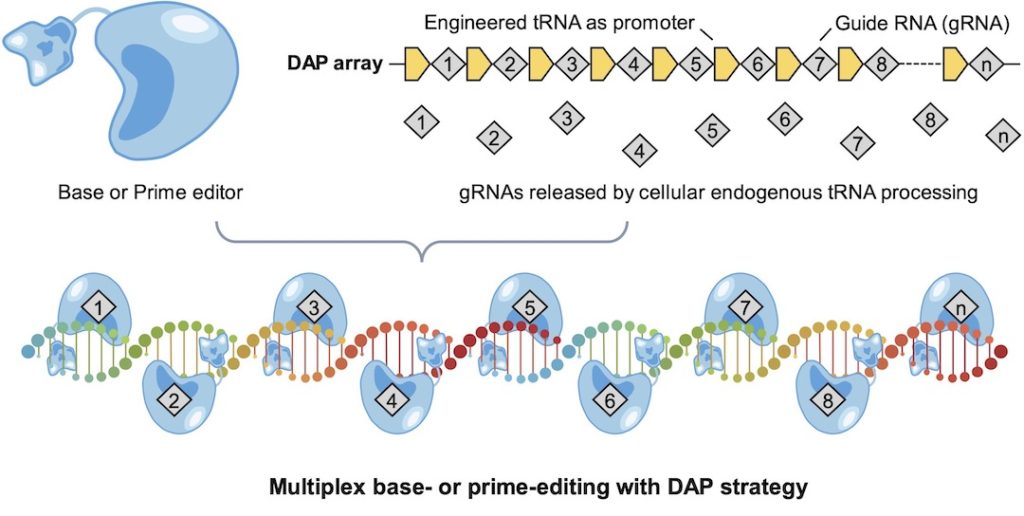
It does so by leveraging transfer RNA (tRNA), a small molecule critical to protein synthesis, as a promoter that drives the expression of multiple guide RNAs (gRNAs) on a single array, then released individually by cells to direct the state-of-art genome editors (base editor or prime editor) for edits at multiple human genomic sites.
In lab experiments, the researchers engineered a short, 75-nucleotide human cysteine tRNA to make highly active DAP arrays that enable up to 31 edits with the base editor and three edits with the prime editor at once. Deliveries of DAP array and genome editors via therapeutic container adeno-associated virus (AAV) or lentivirus were further demonstrated in human cells.
“Previously, if we wanted to edit multiple genes in the same cell, we would have to do them one after another, which is very time-consuming and low-efficiency,” said Yuan, a third-year graduate student in the Gao lab who worked on the project through the COVID-19 pandemic.
“Now, we have a much neater solution,” he said. “For this paper, we demonstrated 31, but in principle with a single DAP array, if not limited by manufacturing and delivery, we could achieve as many edits as we want.”
DAP’s trick is twofold: First, it has no need for the lengthy promoter DNA sequence that initiates multiplexed gRNAs, and second, it’s a plug-and-play solution that accepts guide RNAs for a variety of targets.
“At the very beginning, we sought to demonstrate multiplex base editing with CRISPR-Cas12a, which can release its own guide RNA on a short array,” Yuan said. “However, only low efficiencies were observed.
“Cas9 cannot process its own gRNA array but is favored for efficient base editing,” he said. “We showed that a spacer-like tRNA itself is enough to release multiple Cas9 gRNAs from a compact array without using a lengthy promoter.”
The researchers tested versions of DAP that could collectively install disease-suppressing edits in human cell models against heart disease, Type 2 diabetes, muscular dystrophy, sickle cell disease and beta thalassemia.
Their results showed varying degrees of successful edits and minimal off-target editing, which may be due to releasing just enough gRNA to perform the assigned task.
“Since we are introducing multiple gene edits at once, one could imagine it might lead to more off-target edits,” Gao said. “But our experimental data is very impressive. We actually observed fewer off-target activities while maintaining the same level of on-target editing with DAP arrays.”
“This might be because the individual gRNA concentrations released by DAP arrays could be efficient for the on-target editing but not enough for off-target editing,” she said.
Yuan noted that demonstrating the compatibility of DAP arrays with the two viral vectors most often used in gene therapy, AAV and lentivirus, should help move it toward in vivo studies.
“We anticipate we could pair DAP arrays with base editors, prime editors and other emerging CRISPR technologies, such as multiplex CRISPR screening and studying of polygenic diseases in vivo,” Gao said. “Our lab has a current focus using these technologies for the disease modeling and treatment of cystic fibrosis.”
Gao is the T.N. Law Assistant Professor of Chemical and Biomolecular Engineering and an assistant professor of chemistry and bioengineering.
The National Institutes of Health (R01 HL157714) supported the research. https://news.rice.edu/news/2022/dap-array-casts-wide-net-fix-mutations






Recent Comments