Nerve cells in the brain demand an enormous amount of energy to survive and maintain their connections for communicating with other nerve cells. In Alzheimer’s disease, the ability to make energy is compromised, and the connections between nerve cells (called synapses) eventually come apart and wither, causing new memories to fade and fail.
A Scripps Research team, reporting in the journal Advanced Science, has now identified the energetic reactions in brain cells that malfunction and lead to neurodegeneration. By using a small molecule to address the malfunction, which occurred in the mitochondria—the cell’s major energy producers—the researchers showed that many neuron-to-neuron connections were successfully restored in nerve cell models derived from human Alzheimer’s patient stem cells. These findings highlight that improving mitochondrial metabolism could be a promising therapeutic target for Alzheimer’s and related disorders.
“We thought that if we could repair metabolic activity in the mitochondria, maybe we could salvage the energy production,” says senior author Stuart Lipton, MD, Ph.D., Step Family Foundation Endowed Professor and Co-Director of the Neurodegeneration New Medicines Center at Scripps Research, and a clinical neurologist in La Jolla, Calif. “In using human neurons derived from people with Alzheimer’s, protecting the energy levels was sufficient to rescue a large number of neuronal connections.”
In the new study, Lipton and his team found a block in the enzymes that make energy due to an abnormal tag of nitrogen (N) and oxygen (O) atoms onto a sulfur (S) atom, all together forming a dysfunctional “SNO” enzyme. This reaction is termed S-nitrosylation, and the team demonstrated that a virtual “SNO-Storm” of these reactions occurred in the Alzheimer’s brain neurons.
Lipton and his colleagues initially made the discovery of the “SNO-tag” on energy enzymes by comparing human brains (obtained at autopsy from people with Alzheimer’s disease) to those with no brain disease. The researchers subsequently generated nerve cells from stem cells derived from skin biopsies of people with and without a genetic mutation that causes Alzheimer’s disease. Then, using a series of metabolic labels and an oxygen-measuring apparatus, they calculated cellular energy production and identified unique deficits in the Alzheimer’s nerve cells compared to controls.
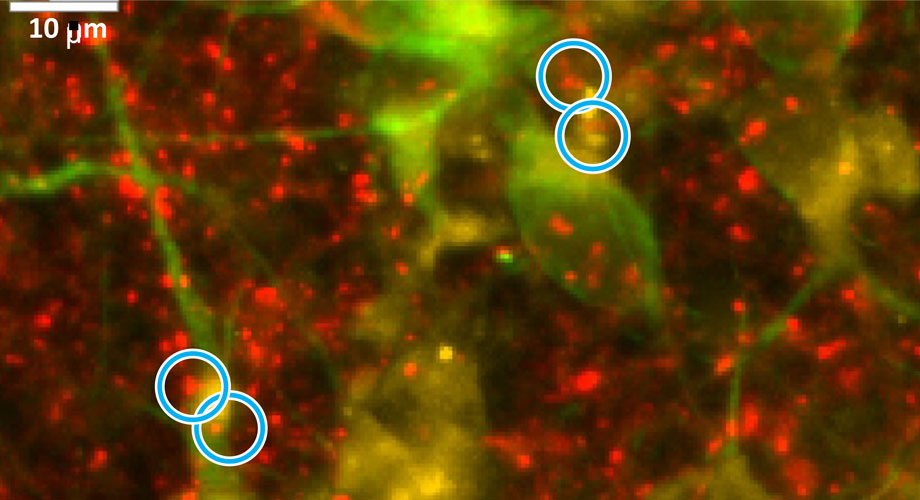
The researchers found that the neurons had a disrupted Krebs cycle—the cellular process in mitochondria that produces most of the body’s crucial molecular power source, ATP. The team pinpointed a bottleneck (or block) in the step where a key molecule is formed: succinate, which drives much of the subsequent production of ATP. In the study, the bottleneck inhibited the mitochondria’s ability to produce the energy needed to sustain neurons and their numerous connections.
The researchers hypothesized that if they could supply some of the missing succinate molecules, they might be able to restore energy production—essentially jumpstarting the stalled mitochondrial Krebs cycle. Since succinate does not easily travel in or out of cells, they used an analog that could better pass through nerve cell membranes. The strategy worked, repairing up to three quarters of the synapses that had been lost, while preventing further decline. https://www.scripps.edu/news-and-events/press-room/2024/20240102-lipton-alzheimers.html







Recent Comments