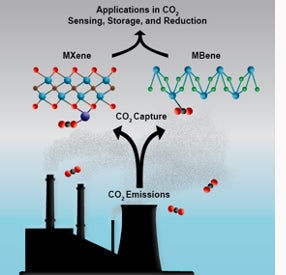
MXene and MBene compounds hold promise for new technologies to combat climate change. Some of the thinnest materials known to humankind — MXene and MBene compounds — may provide solutions to scientists in their quest to curb the effects of global warming. These substances are only a few atoms thick, making them two-dimensional. Because of their large surface area, the materials have the potential to absorb CO2 molecules from the atmosphere, which could help reduce the harmful effects of climate change by safely sequestering carbon dioxide, according to a review study.
Some of the thinnest materials known to humankind may provide solutions to scientists in their quest to curb the effects of global warming.
Known as MXene and MBene compounds, these substances are only a few atoms thick, making them two-dimensional. Because of their large surface area, the materials have the potential to absorb carbon dioxide molecules from the atmosphere, which could help reduce the harmful effects of climate change by safely sequestering carbon dioxide.
In a paper published Oct. 4 in the journal Chem, UC Riverside professor Mihri Ozkan and her co-authors explain the potential of MXenes and MBenes in carbon capture technologies.
“In this review, we conducted an exhaustive analysis and proposed strategies for the widespread implementation of these materials in large-scale applications,” said Ozkan, a climate action professor in UCR’s Electrical and Computer Engineering Department at the Bourns College of Engineering.
“Their unique properties make them excellent candidates for capturing carbon dioxide.”
According to Ozkan, these two-dimensional materials can be engineered to selectively capture carbon dioxide. One of their key advantages is their high selectivity towards carbon dioxide, which can be attributed to a process called interlayer distance engineering. Additionally, the materials are mechanically stable and maintain their structural integrity even after multiple cycles of carbon capture and release.
As human-caused carbon dioxide emissions continue to increase, developing carbon-capture technologies has become a top priority. It is projected that the planet’s temperature could rise by 1.5°C above pre-industrial levels within the next decade, leading to more frequent severe weather events, worsening drought, crop failures, increased levels of human migration, and political instability. These negative impacts highlight the urgent need for action to curb carbon emissions and mitigate the effects of climate change.
Scientists at Drexel University in Philadelphia, Pa., discovered MXenes and MBenes in the early 2010s. MXene is an inorganic compound made up of atomically thin layers of transition metal carbides, nitrides or carbonitrides. On the other hand, MBenes are dimensional transition metal borides made from boron. These compounds are produced through chemical etching techniques and have crystalline lattices with repeating orthorhombic and hexagonal structures.
Ozkan explained that these materials can be used in conjunction with existing technologies, such as those developed by the Swiss company Climework AS. These systems extract carbon dioxide directly from the atmosphere and sequester it for safe and long-term storage.
Before these compounds can be used in carbon capture devices, several technical issues need to be resolved, according to Ozkan. First and foremost, scientists must address the bottlenecks associated with synthesis-related challenges in large-volume production. Other obstacles to large-scale manufacturing include non-uniform mixing, temperature gradients, and problems with heat transfer, among others.
Still, these hurdles can be overcome.
A top-down approach is ideal for large-scale MXene synthesis by scaling up wet etching methods or developing new ones, according to Ozkan.
The paper’s co-authors are UCR’s Kathrine A.M. Quiros, Jordyn M. Watkins, Talyah M. Nelson, Navindra D. Singh, Mahbub Chowdhury, Thrayesh Namboodiri, Kamal R. Talluri, and Emma Yuan. https://news.ucr.edu/articles/2023/10/04/two-dimensional-compounds-can-capture-carbon-air







Recent Comments